Chemistry Pathways
Chemistry Unit 1

Overview
The development and use of materials for specific purposes is an important human endeavour. In this unit you will investigate the chemical structures and properties of a range of materials, including covalent compounds, metals, ionic compounds and polymers. They are introduced to ways that chemical quantities are measured. They consider how manufacturing innovations lead to more sustainable products being produced for society through the use of renewable raw materials and a transition from a linear economy towards a circular economy.
You will conduct practical investigations involving the reactivity series of metals, separation of mixtures by chromatography, use of precipitation reactions to identify ionic compounds, determination of empirical formulas, and synthesis of polymers.
Throughout this unit you will use chemistry terminology including symbols, formulas, chemical nomenclature and equations to represent and explain observations and data from their own investigations and to evaluate the chemistry-based claims of others.
A student-directed research investigation into the sustainable production or use of a selected material is to be undertaken in Area of Study 3. The investigation explores how sustainability factors such as green chemistry principles and the transition to a circular economy are considered in the production of materials to ensure minimum toxicity and impacts on human health and the environment. The investigation draws on key knowledge and key science skills from Area of Study 1 and/or Area of Study 2.
Link to > VCE Chemistry Study Design
Prerequisites
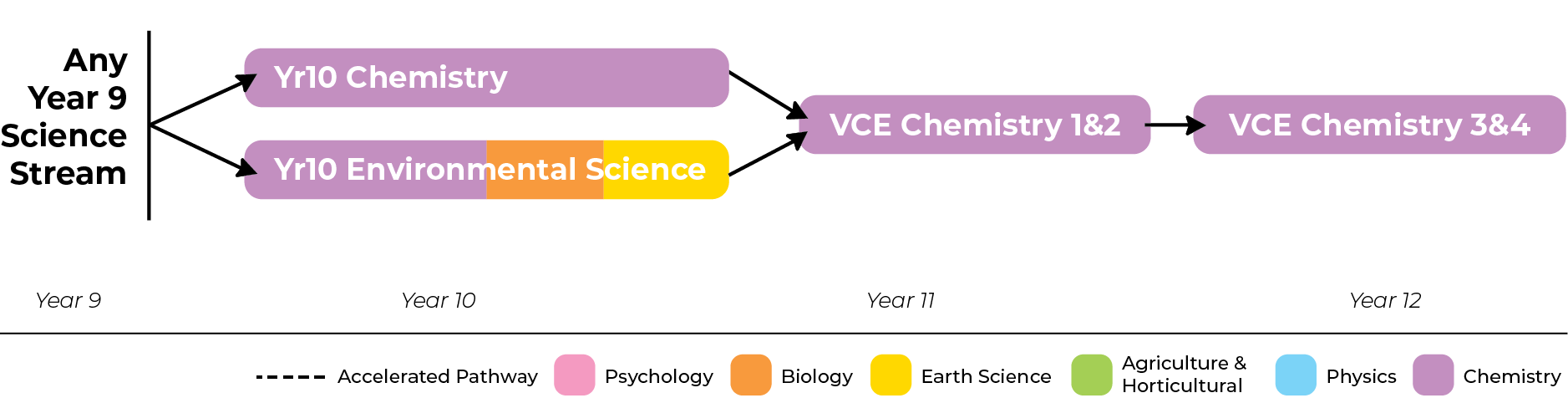
It is expected that students undertaking Units 1 & 2 Chemistry will have studied either Chemistry or Environmental Science at Year 10. Competency in Chemistry topics at the Year 10 level is presumed for Units 1 & 2.
Areas of Study
- How do the chemical structures of materials explain their properties and reactions?
- How are materials quantified and classified?
- How can chemical principles be applied to create a more sustainable future?
In the first area of study, you will focus on elements as the building blocks of useful materials. You will investigate the structures, properties and reactions of carbon compounds, metals and ionic compounds, and use chromatography to separate the components of mixtures. You will use metal recycling as a context to explore the transition in manufacturing processes from a linear economy to a circular economy.
In the second area of study you will focus on the measurement of quantities in chemistry and the structures and properties of organic compounds, including polymers.
In the third area of study you will undertake an investigation involving the selection and evaluation of a recent discovery, innovation, advance, case study, issue or challenge linked to the knowledge and skills developed in Unit 1 Area of Study 1 and/or Area of Study 2, including consideration of sustainability concepts (green chemistry principles, sustainable development and the transition towards a circular economy).
Assessment
Assessment for Outcomes 1 and 2 may include: annotations of a practical work folio; a report of a practical activity; media response; problem-solving; data analysis; or a test.
Outcome 3 will be assessed by a response to a question involving the production or use of a selected material, including reference to sustainability.
Pathways
Chemistry Unit 1 and 2 are completed in Year 11. It is expected that students studying Unit 3 and 4 Chemistry have previously achieved a competent standard in both Unit 1 and Unit 2 Chemistry. Acceleration is not available in this subject.
Chemistry Unit 2

Overview
Society is dependent on the work of chemists to analyse the materials and products in everyday use. In this unit you will analyse and compare different substances dissolved in water and the gases that may be produced in chemical reactions. You will explore applications of acid-base and redox reactions in society.
You will conduct practical investigations involving the specific heat capacity of water, acid-base and redox reactions, solubility, molar volume of a gas, volumetric analysis, and the use of a calibration curve.
Throughout the unit you will use chemistry terminology, including symbols, formulas, chemical nomenclature and equations, to represent and explain observations and data from their own investigations and to evaluate the chemistry-based claims of others.
A student-adapted or student-designed scientific investigation is undertaken in Area of Study 3. The investigation involves the generation of primary data and is related to the production of gases, acid-base or redox reactions, or the analysis of substances in water. It draws on the key science skills and key knowledge from Unit 2 Area of Study 1 and/or Area of Study 2.
Link to > VCE Chemistry Study Design
Prerequisites
It is expected that students undertaking Units 1 & 2 Chemistry will have studied either Chemistry or Environmental Science at Year 10. Competency in Chemistry topics at the Year 10 level is presumed for Units 1 & 2.
Areas of Study
- How do chemicals interact with water?
- How are chemicals measured and analysed?
- How do quantitative scientific investigations develop our understanding of chemical reactions?
In the first area of study you will focus on understanding the properties of water and investigating acid-base and redox reactions. You explore water’s properties, including its density, specific heat capacity and latent heat of vaporisation. You write equations for acid-base and redox reactions, and apply concepts including pH as a measure of acidity. They explore applications of acid-base reactions and redox reactions in society.
In the second area of study you will focus on the analysis and quantification of chemical reactions involving acids, bases, salts and gases. You will measure the solubility of substances in water, explore the relationship between solubility and temperature using solubility curves, and learn to predict when a solute will dissolve or crystallise out of solution. You will quantify amounts in chemistry using volumetric analysis, application of the ideal gas equation, stoichiometry and calibration curves.
The selection of learning contexts should allow you to develop practical techniques to investigate substances that may be dissolved in water or found in soils, particularly salts, acids and bases, as well as gases. You will develop your skills in the use of scientific equipment and apparatus. You will use precipitation reactions to purify water: for example, by using iron or aluminium compounds to precipitate and remove phosphorus from wastewater. You will perform acid-base titrations, such as comparing the ethanoic acid concentrations of vinegar, mayonnaise and tomato sauce. You will construct calibration curves to analyse unknown concentrations of substances, such as the amount of nitrates or phosphates in water or soil samples. You will respond to challenges such as determining the set of standards required in setting up a calibration curve in colorimetry.
Many of the 17 goals in the United Nations’ 2030 Agenda for Sustainable Development relate to ensuring that people have access to potable water, clean air and good quality soil to meet their basic needs. The quality of water, air and soil must be monitored closely to ensure that human health and the environment are not compromised.
In this area of study you will adapt or design and then conduct a scientific investigation related to chemical equations and/or analysis, which must include the generation of primary data. You will develop a research question related to the production of gases, acid-base or redox reactions or the analysis of substances in water, and adapt or design and then conduct a scientific investigation to generate appropriate quantitative data. You will organise and interpret the data and reach a conclusion in response to your research question.
Assessment
Assessment for Outcomes 1 and 2 may include: annotations of a practical work folio; a report of a practical activity; media response; problem-solving; data analysis; or a test.
Outcome 3 will be assessed by a report of a student-designed quantitative laboratory investigation using a scientific poster.
Pathways
Chemistry Unit 1 and 2 are completed in Year 11. It is expected that students studying Unit 3 and 4 Chemistry have previously achieved a competent standard in both Unit 1 and Unit 2 Chemistry. Acceleration is not available in this subject.
Chemistry Unit 3


Overview
The global demand for energy and materials is increasing with world population growth. In this unit you will investigate the chemical production of energy and materials. You will explore how innovation, design and sustainability principles and concepts can be applied to produce energy and materials while minimising possible harmful effects of production on human health and the environment.
You will analyse and compare different fuels as energy sources for society, with reference to the energy transformations and chemical reactions involved, energy efficiencies, environmental impacts and potential applications. You will explore food in the context of supplying energy in living systems. The purpose, design and operating principles of galvanic cells, fuel cells, rechargeable cells and electrolytic cells are considered when evaluating their suitability for supplying society’s needs for energy and materials. You will evaluate chemical processes with reference to factors that influence their reaction rates and extent. You will investigate how the rate of a reaction can be controlled so that it occurs at the optimum rate while avoiding unwanted side reactions and by-products. You will conduct practical investigations involving thermochemistry, redox reactions, electrochemical cells, reaction rates and equilibrium systems.
Throughout the unit you will use chemistry terminology, including symbols, formulas, chemical nomenclature and equations, to represent and explain observations and data from your own investigations and to evaluate the chemistry-based claims of others.
Link to > VCE Chemistry Study Design
Prerequisites
It is expected that students studying Unit 3 and 4 Chemistry have previously achieved a competent standard in both Unit 1 and Unit 2 Chemistry.
Areas of Study
- What are the current and future options for supplying energy?
- How can the rate and yield of chemical reactions be optimised?
Unit Assessment
- Unit 3 School assessed coursework (SAC’s) 20%
- Unit 4 School assessed coursework (SAC’s) 20%
- Unit 3 and/or 4 Practical Investigation 10%
- Unit 3 & 4 examination 50%
Assessment for Outcomes 1 and 2 may include: a comparison of two practical activities; analysis of an innovation or media communication; problem-solving; or data analysis
Unit 3 or 4 Practical Investigation
A student-designed scientific investigation involving the generation of primary data related to the production of energy and/or chemicals and/or the analysis or synthesis of organic compounds is undertaken in either Unit 3 or Unit 4, or across both Units 3 and 4, and is assessed in Unit 4 Outcome 3. The design, analysis and findings of the investigation are presented in a scientific poster format.
Pathways
Acceleration is not available in this subject.
Chemistry Unit 4
Overview
How are carbon-based compounds designed for purpose?
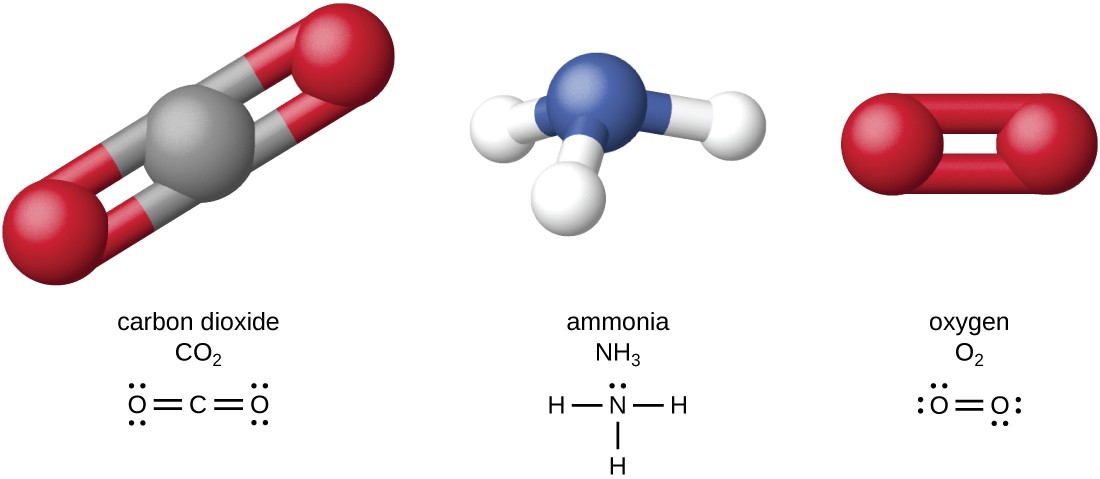
Carbon is the basis not only of the structure of living tissues but is also found in fuels, foods, medicines, polymers and many other materials that we use in everyday life. In this unit you will investigate the structures and reactions of carbon-based organic compounds, including considering how green chemistry principles are applied in the production of synthetic organic compounds. You will study the metabolism of food and the action of medicines in the body. You will explore how laboratory analysis and various instrumentation techniques can be applied to analyse organic compounds in order to identify them and to ensure product purity.
You will conduct practical investigations related to the synthesis and analysis of organic compounds, involving reaction pathways, organic synthesis, identification of functional groups, direct redox titrations, solvent extraction and distillations.
Throughout the unit you will use chemistry terminology including symbols, formulas, chemical nomenclature and equations to represent and explain observations and data from their own investigations and to evaluate the chemistry-based claims of others.
A student-designed scientific investigation involving the generation of primary data related to the production of energy and/or chemicals and/or the analysis or synthesis of organic compounds is undertaken in either Unit 3 or Unit 4, or across both Units 3 and 4, and is assessed in Unit 4 Outcome 3. The design, analysis and findings of the investigation are presented in a scientific poster format.
Link to > VCE Chemistry Study Design
Prerequisites
It is expected that students studying Unit 3 and 4 Chemistry have previously achieved a competent standard in both Unit 1 and Unit 2 Chemistry.
Areas of Study
- How are organic compounds categorised and synthesised?
- How are organic compounds analysed and used?
Unit Assessment
- Unit 3 School assessed coursework (SAC’s) 20%
- Unit 4 School assessed coursework (SAC’s) 20%
- Unit 3 and/or 4 Practical Investigation 10%
- Unit 3 & 4 examination 50%
Assessment for Outcomes 1 and 2 may include: a comparison of two practical activities; analysis of an innovation or media communication; problem-solving; or data analysis
Unit 3 or 4 Practical Investigation
A student-designed scientific investigation involving the generation of primary data related to the production of energy and/or chemicals and/or the analysis or synthesis of organic compounds is undertaken in either Unit 3 or Unit 4, or across both Units 3 and 4, and is assessed in Unit 4 Outcome 3. The design, analysis and findings of the investigation are presented in a scientific poster format.

